The effect of alkaline cations on the rheology of kaolin
In this speech, the effect of alkaline cations on the rheology of kaolin has been studied in a wide concentration range, focusing on millimolar concentration levels. The partial dispersion of kaolin with
the help of sodium polyacrylic acid exposed to the addition of alkaline chloride salts has formed the basis of the tests of the present study. As the size of alkaline cations increases, their specific adsorption
on clay surfaces increases significantly. and they change the viscoelastic properties of the coagulated system. The effect of cations becomes important when there are balanced weak anions. However,
agglomerating anions such as borates, silicates and phosphates can also contribute to the dispersion of the clay slip system with the help of specific adsorption on the surface of the clay particles. Increasing the concentration of cations and the effect caused by it will destroy the stability of the suspension. The rheology of dispersed slips with a high solid percentage are sensitive to even a few tens of millimolar fluctuations in the concentration of alkali compounds – which we usually encounter in the production of ceramic bodies. The contact and collision of alkaline cations causes the effects that we usually encounter during production. Kaolin can be considered as a compound of molecular layers of silica and alumina, with one face having silica-like properties and one face having alumina-like properties. The crystal structure of kaolin is composed of a two-layer adjacent system consisting of tetrahedral silica and octahedral aluminum layers. The accumulated layers of these silica-aluminum composite pairs are considered as the basis for the formation of clay particles. The relationship between this structure and the behavior of clay particles as well as the separate behavior of silica and aluminum in aqueous suspensions is one of the efforts of this speech.
Bivalent alkaline earth metals play a very important role in ceramic grouts. The critical coagulation of divalent calcium and magnesium appears only in the concentration range of about one-tenth of the concentration of monovalent alkaline cations, and this range is well covered by the fluctuations of the hardness of the water used, and therefore leaves significant effects on the rheological behavior of slips.
The contribution of monovalent alkaline cations in such slips is completely influenced by divalent cations.
However, in order to understand the behavioral nature of kaolin, we have to study the role of monovalent alkaline salts as well.
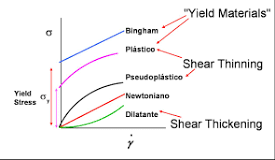
By studying the resulting flow curves for each suspension, we observe a linear region in the log-log scale of shear rate according to viscosity. The slope of this linear region, as well as its sequence, which has a high shear rate, is the same for all suspensions. Indexing each flow curve with the help of apparent
viscosity at a certain shear rate (1/S) is an effective method to check the condition of each slurry. Drawing the apparent viscosity curve according to the concentration of the added metal cation while each suspension contains 18.4 mmol of cation (Na^+) from the lubricant, and subsequently fiƫng the concentration curve according to the apparent viscosity data using a uniform transfer function, and Calculating the root of the second derivative of the resulting curves can determine the point of curvature of changes in the viscosity index. This turning point of the curve is appropriately determined with the help of the “critical freezing point” of each series of alkalis. The results clearly show that monovalent alkaline metals in concentrations higher than their threshold level have a strong coagulation effect on kaolin. Each of the alkaline chlorides has a concentration threshold at which the viscosity of the kaolin slurry increases sharply and rapidly in parallel with the increase in concentration. The final state of the coagulated slurries shows subtle differences for each metal cation. However, the amount of “critical viscosity” of the metal cation, where the viscosity changes rapidly, is remarkably the same for all alkali metal cations. In this study, each suspension contained 18.4 mmol [Na^+] obtained from sodium polyacrylate acid (NaPAA), which was used as a lubricant. The slurries reached their “critical viscosity concentration” when approximately 7 mmol of additional cations [M^+] were introduced into the system, which resulted in an increase in the concentration of alkaline compounds to 25 mmol. There is a determined crisis. The mentioned differences indicate that the “critical opacity concentration” decreases to some extent with increasing atomic number or ionic radius. Researches have shown that the effect of magnesium and calcium cations in similar systems is almost additive and they intensify each other’s effect. The addition of cations is always accompanied by the entry of charge-balancing anions. Each alkaline metal holds a single positive charge. Each hydrated metal cation also occupies almost the same volume in the solvent space and obtains similar access to the surface of clays due to electrostatic forces.
The viscosity of dispersed kaolin slurries is achieved by neutralizing (at least partially) the negative charges of the silica base surfaces of the clay. negative charges that cause repulsion between particles
and their separation from each other. They analyze the behavior of kaolin towards ionic salts and polyanionic lubricants based on its structure and molecular chemistry. Colloidal silica particles acquire a negative charge due to the acidic structure of the silica surface near neutral pH. The closed bridge oxides located on the surface react with water and form acidic silanol groups. The acid-base balance of silanol groups causes a negative charge on silicate particles and free protons. On the contrary, the alumina surface of kaolin particles should absorb protons in aqueous suspensions and have a positive charge near neutral pH.
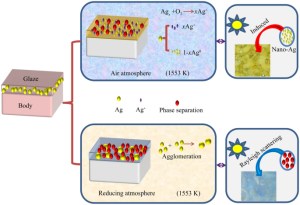
The application of the chemistry of noble metal nanoparticles in the preparation of digital printing inks
The application of the chemistry of noble metal nanoparticles in the preparation of digital printing inks In recent years, the field of nanotechnology and nanoparticle